In the late 1800s, Dr. William Coley—a bone surgeon and cancer researcher at New York Cancer Hospital—observed something peculiar. A patient named Fred Stein was suffering from a tumor growing in his cheek—until he became infected by Streptococcus pyogenes bacteria (which causes strep throat). Shortly after his infection, the cancer began disappearing, as though the fever had burned it away.
Afterward, Coley began to notice that several other cancer patients who had recently undergone tumor-removal surgery were more likely to recover from their cancer if they developed a post-surgical infection. In an effort to figure out why, Coley began injecting inoperable cancer patients with streptococcal bacteria. These came to be known as "Coley toxins." In one case, Coley treated a 21-year-old man with a mix of bacteria and bacterial lysates—natural secretions of bacteria that keep the immune system on alert—who then had a complete remission.
Coley injected over 1,000 patients with his toxins—and many recovered. But he never properly documented all his cases or followed up with enough patients, and after his death in 1936, general medical opinion dismissed his methods in favor of radiation and chemotherapy. It wasn't until much later, when several pioneering cancer researchers revisited his work, that the medical community began to realize that Coley—sometimes called the "father of immunotherapy"—had been onto something.
In the fall of 2014, the FDA approved an immunotherapy drug known as Anti-PD1 for melanoma, the most serious type of skin cancer. Soon after, Anti-PD1 became the standard of care for melanoma. It's so effective, in fact, that it's used completely alone, without the need for chemotherapy or radiation. "I have not given chemotherapy to a person with melanoma for the past two years," says Dr. Antoni Ribas, a medical oncologist at UCLA who treats mainly melanoma patients. "The days of chemotherapy for these diseases are over."
Anti-PD1, like all immunotherapies, works by hacking your immune system—essentially, teaching it how to attack cancer cells, which it would otherwise ignore. There are huge advantages to immunotherapy compared with traditional cancer treatments. When patients undergo chemotherapy, the side effects are often debilitating, including extreme pain and fatigue, nausea, diarrhea, hair loss, poor appetite and a risk for life-threatening infections, as well as long-term health consequences like heart and lung disease. In addition, chemotherapy and radiation generally don't guarantee lasting protection from recurrence.
Immunotherapy, on the other hand, "would get the immune system to impact cancer long-term, because the immune system has the ability to remember," says Ribas. "So if you develop a therapy that turns on the immune system correctly, it will continue to remember that the bad guy is the tumor and should be attacked."
That's why the field of immunotherapy research has exploded in recent years. And one of the most promising areas of cancer immunotherapy goes all the way back to Coley: Controlled bacteria might be the best tool yet to turn the immune system into a cancer-fighting machine.
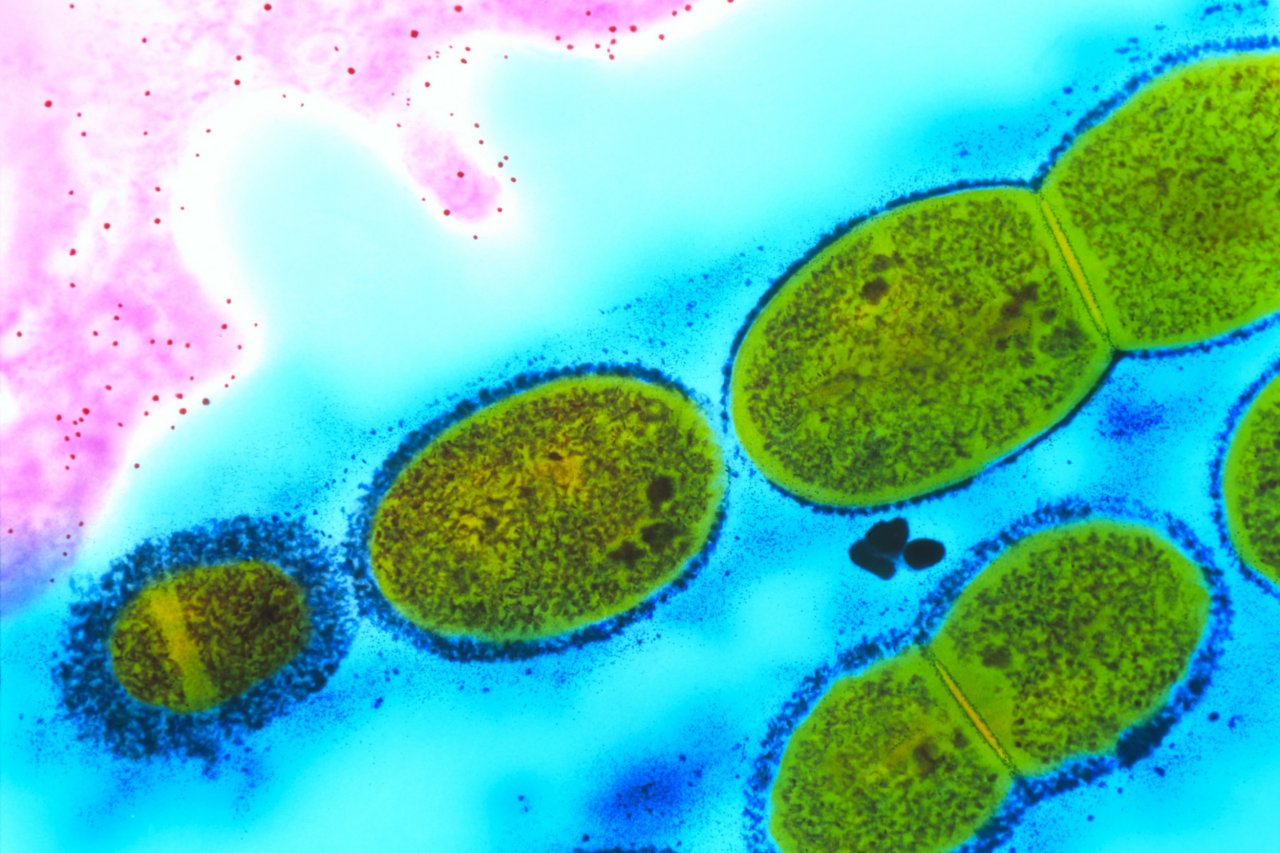
We know Salmonella bacteria as a sickening bug, lurking in undercooked meat or buckets of cookie dough and making its way into our system if we don't prepare our food properly. When it does, it wreaks havoc on us in the form of nausea, fever, diarrhea, vomiting and chills. But there's another side of Salmonella.
Roy Curtiss, a professor who runs a lab at the Arizona State University's Biodesign Institute has been studying the bacteria's cancer-killing properties for some time now. He's found that certain strains of Salmonella, when genetically modified to become safer, have the ability to enter cancer cells and take over. It's different from anti-PD1 therapy, where the immune system is taught to recognize cancer cells that were previously "hidden"—with Salmonella, the bacteria itself can exert its toxic effects on individual tumor cells.
But there's been one major challenge in introducing the Salmonella cure to humans: The bacteria are toxic and can cause infections and even sepsis, especially if the person's immune system is compromised. "You kill the tumors, but then you kill the patient," says Curtiss. "It's a struggle to find a balance between the bacteria's ability to reach a solid tumor and multiply profusely there in the manner that ultimately kills most of the tumor cells—and the ability to prevent damage to healthy tissues."
Curtiss's most recent project involved genetically modifying Salmonella to lower the toxicity of the bacteria while maintaining its efficacy. To do so, Curtiss and his research team altered its lipopolysaccharide structure, or outer membrane, which is the primary culprit in causing sepsis. They fine-tuned a little more, then injected the bacteria into mice with tumors. It turned out to be a rousing success, killing the tumors without harming the healthy cells nearby. This proved, for the first time, that bacteria could fight cancer without any serious side effects.
Salmonella is one of the few strains of bacteria, along with Listeria and Clostridia, that have shown potential in entering, colonizing and ultimately destroying cancer cells. One member of the Clostridia group, Clostridium novyi, is particularly promising. In 2014, researchers at Johns Hopkins University injected a modified version of the bacteria, called C. novyi-NT, into cancer-stricken dogs and found it could reduce their tumors. They even tried it out, successfully, on one human patient with advanced leiomyosarcoma—a rare form of smooth muscle cancer.
C. novyi-NT is unique because it thrives in a low-oxygen environment—and the centers of tumors, it turns out, have very little oxygen. Once injected into the tumor, the bacteria are "in a low-oxygen environment, where they germinate, begin to divide and grow, and in the process consume cancer cells," says David Chao, president and CEO of BioMed Valley Discoveries, which is collaborating with Johns Hopkins. The bacteria then stop growing at the rim of the tumor, where there is more oxygen to be found—preventing them from going any further into healthy cells.
Larger clinical trials of C. novyi-NT on humans are now in the works. It's difficult, of course, to predict how well treatments that have been successful in animal trials will translate to people. Dr. Mario Sznol, at Yale University, worked on Salmonella research for five years only to find that exciting results in rats and dogs didn't occur in tests on human tissue. "What we learned is that we don't see the same kind of tissue colonization [in humans] that we did in mice and rats," says Sznol. "There's something really different about the biology of human tumors." If this obstacle is overcome, he says, bacteria can truly become "nifty" vehicles of tumor destruction.
After Coley died, his daughter, Helen Coley Nauts, fought for years to bring his work to the attention of the medical community. And for years, she was shunned; her father's results were dismissed as lacking in evidence. But she worked tirelessly to organize his data and track down patients who had been treated with Coley's toxins. Even though she wasn't trained as a scientist and hadn't even graduated from college, Coley Nauts ultimately laid the basis for a field of research that now spans across countless labs and pharmaceutical companies, and is flying forward. There are projects developing immune-triggering therapies for lung, breast, colon, head and neck, skin and pretty much every other type of cancer out there.
"In the future," Sznol says, "I think we're going to get so good at it, we're going to actually be able to give patients very limited therapy and cure them of their cancer."
This article is one in a series from Newsweek 's 2015 Cancer issue, exploring challenges and innovations in cancer treatment and research. The complete issue is available online and at newsstands.