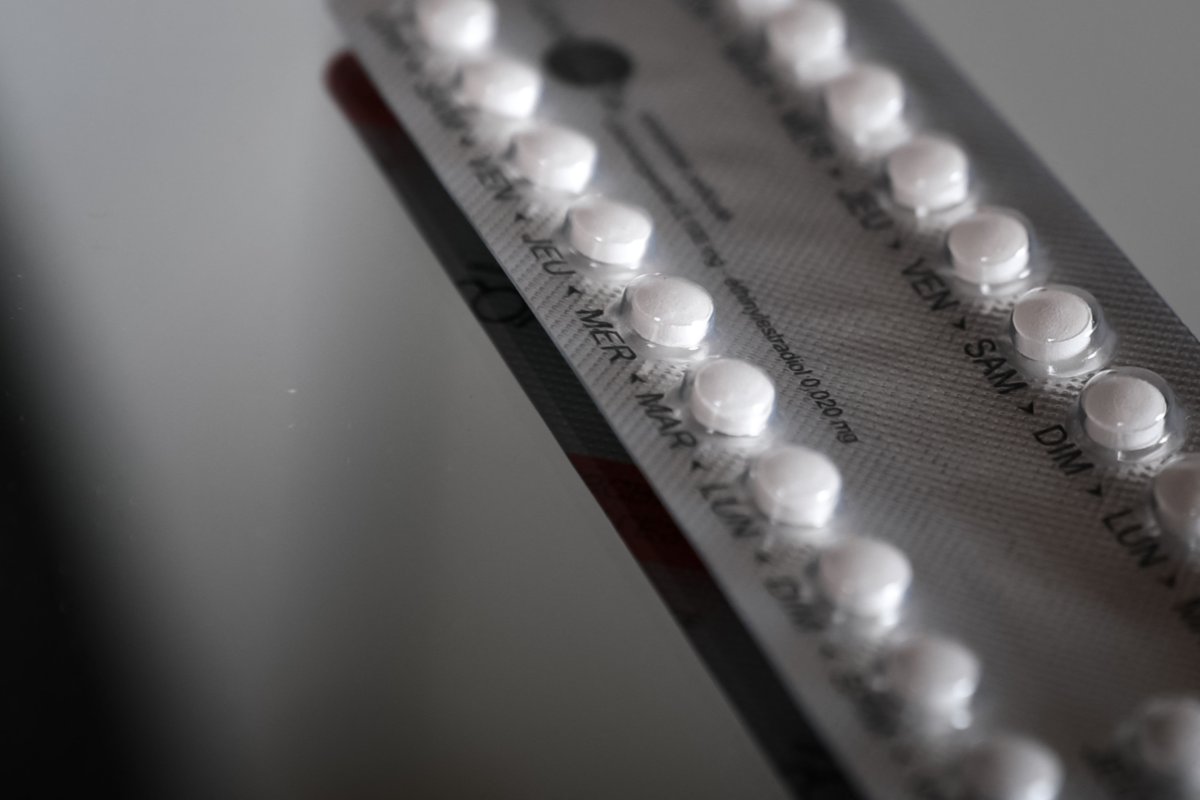
Thousands of Tydemy birth control pills were recalled by the U.S. Food & Drug Administration on August 1 because of concerns over their effectiveness, which the agency said could lead to "unexpected" pregnancies.
The FDA announced that two lots of the Tydemy pill, a prescription oral contraceptive manufactured by Lupin Pharmaceuticals, "may have reduced effectiveness due to decreased levels of ascorbic acid," one of the ingredients contained in the product. Ascorbic acid is most commonly known as vitamin C.
Tydemy is a combination hormone medication that contains drospirenone (a progestin hormone), ethinyl estradiol (an estrogen), and levomefolate calcium, according to the FDA.
Lupin voluntarily recalled the two lots on July 29, advising customers to continue taking their medication but immediately get in touch with their pharmacist, physician, or medical provider for advice over using an alternative contraceptive method. The company said it was recalling the products because of a reduced level of ascorbic acid and high levels of a "known impurity," though this was not specified.
Newsweek contacted Lupin for comment by email on Thursday.
While the FDA has warned that lower levels of ascorbic acid in the two lots could result "in unexpected pregnancy," the agency said that, to date, it has not received any reports of "adverse events" related to using the Tydemy pill. Lupin said the same.
How To Know If You're Affected
If you think you might be in possession of a product from the two recalled batches, check the lot numbers listed on the packaging. The two recalled lots are L200183 and L201560. These were distributed in U.S. pharmacies and supermarkets between June 3, 2022, and May 31, 2023.
If you have taken the tablets in one of the two lots recalled by Lupin and the FDA, the agency said you should report any adverse reactions to the MedWatch Adverse Event Reporting program by completing and submitting a report online or by downloading and completing a form before submitting it via fax at 1-800-FDA-0178.
If you are wondering whether you could get a refund, contact Inmarx Rx Solutions Inc. at 866-480-8206 between Monday and Friday from 9 a.m. to 5 p.m. ET.
According to the FDA's database, the agency has recalled a total of 4,179 boxes of Tydemy to date.
In December 2022, Lupin voluntarily recalled four batches of the blood pressure medication Quinapril after the potential presence of an impurity was identified.
Lupin discontinued the marketing of Quinapril tablets in September 2022.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Giulia Carbonaro is a Newsweek Reporter based in London, U.K. Her focus is on U.S. and European politics, global affairs ... Read more





