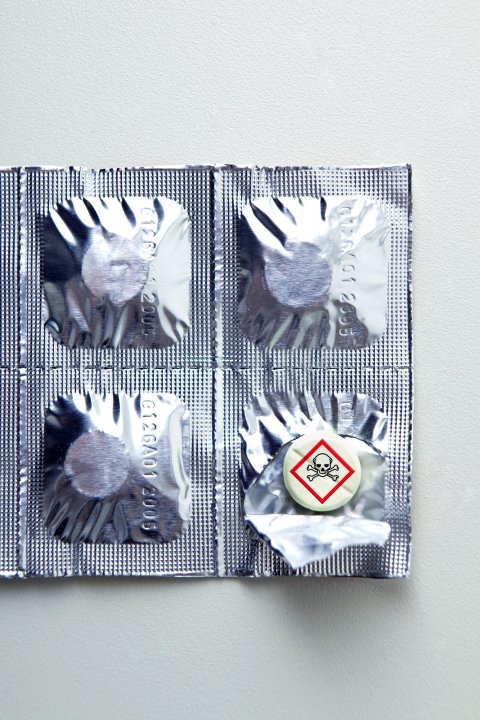
In the mid-2000s, Myanmar saw between 500,000 and 600,000 cases of malaria every year. So it wasn't surprising when, in February 2005, a 23-year-old man in Myanmar came down with a fever, nausea, chills and a headache so severe he had to be taken to the local hospital. His doctors quickly determined he was, in fact, stricken with malaria. They prescribed him artesunate, an inexpensive anti-malarial regularly used by Myanmar's health care professionals to treat the infectious disease.
Typically, a patient's symptoms will subside after a few days on the drug, but this young man grew much worse. He slipped into a coma, his kidneys showed signs of failing, and the concentration of malarial parasites in his blood grew higher. His doctors tried to give him fluids and a more powerful dose of artesunate injected into his bloodstream, but they were too late. The infection spread to his brain and killed him.
Because artesunate is safe, generally well-tolerated and highly effective, hospital investigators decided to probe the case to try to understand what might have gone wrong. They were shocked to discover that the artesunate given to the patient had only 20 percent of the active ingredient required to kill the parasites. The drug, in other words, was a fake.
In the small village, word spread quickly of the tragedy, and community leaders were distraught. No one in their small town had ever died from a fake drug before—at least, not that they knew of. Fearing the threat of other preventable deaths, they collected all the artesunate from the hospital's supply, went out to local pharmacies and pulled any other suspicious artesunate from the shelves, and then publicly burned it all.
Tragic incidents like this happen all over the world and with almost every type of drug. In Pakistan, a poor-quality tuberculosis drug killed 100 patients at a Lahore hospital in 2012 by triggering severe adverse reactions. In 2013, officials in India discovered that 8,000 patients died over a five-year period in a remote Himalayan hospital because an antibiotic used to prevent infection after surgery had no active ingredient. And in May this year, the World Health Organization (WHO) issued a warning about expired meningitis vaccines being sold in West Africa—a devastating blow to those trying to slow a viral outbreak in the region.
Every type of drug is susceptible, from anti-malarials to vaccines, antibiotics, HIV treatments and even Viagra. At their best, falsified drugs have none or too little of the active ingredient; at their worst, sellers are providing hospitals and patients with "drugs" that are life-threatening poisons.
"These falsifiers are in fact murderers—they are causing death," says Jim Herrington, executive director of the Gillings Global Gateway at the University of North Carolina's Gillings School of Public Health. "And you're more likely to get prosecuted for counterfeiting a Gucci purse than a drug."
Internet Roulette
Task forces are finding more fake drugs every year. Interpol's flagship pharmaceutical investigation, Operation Pangea, for example, says it seized 2.4 million fake and illicit pills in 2011; in 2015, the total number of pills and other medications that officials seized jumped to 20.7 million.
That's either good news or a terrible harbinger of what's to come. It might be the case that officials finally know where to look for fakes and are just now catching up to the crooks. Though public health officials have known about falsified pharmaceuticals for decades, they didn't understand the extent of the catastrophe until they started collecting data in the early 2000. Interpol's pharmaceutical crime unit wasn't even founded until 2005.
On the other hand, many experts believe that the problem is on the rise and that more criminals are turning to pharmaceuticals for a simple reason: low risk and high reward. "The penalties are relatively weak for trading in falsified pharmaceuticals compared to those for trade in narcotics and human trafficking," says Paul Newton, a professor of tropical medicine at the University of Oxford medical school who has spent decades tracking poor-quality medicines. And criminals can make a lot of money by falsifying drugs that are in high demand, in short supply or are exorbitantly expensive for consumers.
"We've seen it happen regularly—if a shortage occurs, hospitals and clinics will step outside the normal supply chain, and the [criminals] exploit the situation," says Michael Deats, a group lead for the WHO's Department of Essential Medicines and Health Products. Meanwhile, different organizations over the past few years have estimated that anywhere from 100,000 to a million people die every year due to falsified drugs. This number has likely risen over time, echoing what experts assume to be a rise in the number of fake drugs in circulation. But it's impossible to know for sure, in large part because it's so difficult to know that it was, in fact, a fake drug that killed someone. Maybe the diagnosis was wrong. Maybe a quality drug was administered too late.
The global pharma industry has complex networks that crisscross the globe. A single pill might pass through a dozen countries during its manufacturing process, which offers many opportunities for criminals to put fake drugs into the supply chain. For example, chemicals synthesized in China can be combined with fillers in India and then packaged in Mexico before arriving at a pharmacy in Canada. Often, fake-drug-trafficking criminals have extensive international networks. In 2013, a Puerto Rican man was sentenced to two years in prison for selling hundreds of thousands of fake pharmaceuticals online. He was the American contact for a counterfeit drug ring allegedly headed by Bo Jiang, a Chinese national who was last seen in New Zealand before he went on the run from officials.
The international nature of the fake-drug trade is what makes it such a difficult problem to manage. "Very few of the 196 countries in the world have a specific dedicated service to deal with pharmaceuticals," says Aline Plançon, the assistant director of Interpol's program fighting counterfeit medical products and pharmaceutical crime. "Others can't enforce their laws because they don't have the capacity or budget."
When pharmaceutical components arrive at each new country, in most cases enforcement agents check a drug's paper trail to ensure legitimacy and spot-check the packaging, as well as its chemistry and appearance. But criminals often use falsified papers to sneak their fake drugs through checks, and the WHO estimates that about 30 percent of countries worldwide don't have a functional drug regulation agency equivalent to the Food and Drug Administration (FDA) in the U.S.
Not surprisingly, these countries are more likely to be relatively poor, with underfunded and understaffed governments. With limited government oversight, officials in these countries sometimes accept bribes. A 2014 report published by the Independent Joint Anti-Corruption Monitoring and Evaluation Committee found, for example, that in Afghanistan, Ministry of Public Health officials "solicit bribes for activities outside their departmental mandates with little chance of being detected." In China, an official was executed in 2007 for accepting bribes to approve untested medicines.
Even in wealthier countries like the U.S. and the United Kingdom, where drugs are tested frequently, fakes can slip through, often when patients or clinicians buy them over the Internet. Studies show that about 90 percent of drugs purchased online come from a different country than what the website claims, and Internet pharmacies often buy drugs from countries with lax regulatory systems.
The majority of drugs purchased online are sent through the mail. Most of these shipments are subjected to standard border control mechanism, including X-rays and drug-sniffing dogs. If there's a reason to believe a specific shipment is likely to have fakes—for example, if officials have received reports that certain drugs have been falsified recently, or if the paperwork seems suspicious—customs agents are more likely to open the packages and inspect their contents, according to a spokesman from the National Intellectual Property Rights Coordination Center (NIPRCC), one of the government agencies involved in U.S. customs protection.

But neither the FDA nor U.S. Immigration and Customs Enforcement could tell Newsweek what percentage of these drugs are actually checked or confiscated every year. Even if they find something amiss, there's no guarantee they'll seize the product—seizures involve a 25-step process of testing and investigations, the NIPRCC spokesman says, and they are costly in time, manpower and resources. So officials seize drugs only if they are both suspicious and likely to affect a large number of people. " Seizures happen if there's a lot of a product, often thousands of dollars' worth," the NIPRCC spokesman adds.
If there's not enough of a product to conduct a formal seizure, officials set the package aside and send a letter to its intended recipient saying that officials suspect that this product contains fakes and that, if he wants the shipment anyway, he assumes the risk of its contents. If the recipient contacts officials in response to the letter, they will ask questions about where the drugs were purchased so that customs agents can more effectively monitor future shipments. The FDA says that screening and evaluation programs like these have led its Office of Criminal Investigations to arrest more than 400 people over the past two decades.
The system is far from foolproof. In 2011, for example, the FDA received reports that customers who had ordered drugs like Ambien, Xanax and Lexapro online instead received pills of Haldol, a potent antipsychotic. According to the FDA, "These customers needed emergency medical treatment for symptoms such as difficulty in breathing, muscle spasms and muscle stiffness."
Big, Dirty Pharma
Stopping this scourge would at a minimum require a great deal of cooperation among more than a handful of nations. Amir Attaran, a professor of law and medicine at the University of Ottawa, suggests an international treaty whereby countries would all agree on a set of laws. Attaran compares it to the aviation industry: "There are dozens of treaties on civil aviation, and every single country is following those. If not, they don't fly." To raise the standards for pharmaceuticals worldwide, Attaran says, we need a similar system that penalizes countries that don't enforce medicinal quality controls.
The closest protocol now is the Medicrime convention: Since 2011, countries can sign the informal treaty to criminalize pharmaceutical fraud within their borders. But countries aren't under much pressure to pass more formal legislation or to enforce the statutes of the convention. And in fact, they often have incentives not to. Some countries, like India and Brazil, are dragging their feet on international enforcement regulations because poor-quality pharmaceuticals make up such a large part of their economy, Attaran says.
Others are trapped by policies that conflate fake and counterfeit drugs. Counterfeit drugs are those that infringe on a patent registered by the pharmaceutical company. Counterfeit Viagra, for example, might contain the same ingredients as the legitimate drug, but Pfizer didn't authorize the pill's production and doesn't get a cut of the profits. Counterfeit drugs don't necessarily endanger people's lives—they're more a threat to Big Pharma's bottom line than anything else. From a public health perspective, falsified drugs are the real menace, as they kill many thousands of unsuspecting people around the world each year. But in countries where policies do not adequately distinguish between the two, enforcement agencies have to spend their limited resources cracking down on minuscule infringements on intellectual property instead of tracking down the cartels falsifying drugs.
The WHO is in a unique position to resolve the issue. The organization hosts and mediates the conferences in which countries meet to discuss what they can do together to reduce the number of falsified pharmaceuticals reaching patients. It also is the central clearinghouse for reports of fake drugs across the world. But, to date, the organization has declined to push countries to sign fake-drug-related treaties and has not taken a strong stand on separating out the public health issue of falsified drugs from the counterfeit concerns of manufacturers. The WHO has used and will continue to use the term " substandard, spurious, falsely labeled, falsified and counterfeit"—SSFFC—to talk about the larger public health issue, Deats says, "until member states agree on a universal definition." But Attaran and others say that this is really because the WHO doesn't want to alienate Big Pharma—a close partner and financial supporter.
"The difference between falsified and counterfeit drugs seems trivial, but half the reason the world isn't doing more about pharmaceutical crime is precisely because of this language," Attaran says. "Pharma companies, at least dirty ones, have tried to expand the fight against falsified drugs to protect their intellectual properties, and that's just wrong."
A year ago, Attaran was working as a consultant for a project on falsified pharmaceuticals with the United Nations Office of Drugs and Crime. At one point, he says, he was ordered to make sure they would also go after intellectual property cases by someone working at Sanofi, the French pharma giant. Sanofi had explicitly co-sponsored the project through its nonprofit subsidiary, the Institute of Research Against Counterfeit Medicine. (An IRACM spokeswoman confirmed that the organization was founded in 2010 at "the instigation of Sanofi" and that it continues to receive funding from the company.) Attaran withdrew from the project for ethical reasons, and it continued on without him. In December 2014, several member countries sent letters of complaint to the UNODC because its policies linked counterfeit and falsified medicines.

Big Pharma has a lot of sway with the international organizations working to keep drugs safe. The IRACM, for example, partners with a number of global regulatory groups in addition to the U.N., like the World Customs Organization and Interpol. And in recent years, the WHO has been accused by journalists and nonprofits of falling under the influence of Big Pharma after accepting donations from organizations explicitly funded by it; in 2007, The BMJ published an article exposing the fact that a $10,000 donation to the WHO was made by pharma giant GlaxoSmithKline, laundered through the nonprofit European Parkinson's Disease Association.
Asked about the WHO's relationship with pharmaceutical companies, Deats says that in order to combat SSFFC drugs, all kinds of stakeholders need to collaborate on every scale. " That means working with public sector, private sector, health care professionals, civil society and law enforcement." Ultimately, he says, the WHO should not be responsible for a legal framework. He sees his organization as a facilitator, a means through which countries and health care professionals can communicate when they find fake drugs. But the legal framework as well as the enforcement of those laws, he says, are out of the WHO's hands—and up to individual countries.
There is one group acting as an international regulator for fake drugs. In the past seven years, Interpol's Operation Pangea has led to the seizure of millions of packages of falsified drugs, many of which were sold online. This is always touted as a great example of successful international collaboration—one Pangea bust in 2014 involved authorities from 111 different countries, according to an FDA press release at the time.
But those numbers are deceiving, Attaran says. "What Interpol never tells you is that well over half the medicines they seize in Pangea operations are from only a few countries, such as the United Kingdom, the U.S. and France"—countries that already have strong regulatory and enforcement systems. "It's really an isolated few national efforts stitched together by Interpol to create the illusion of a grand global effort [against falsified pharmaceuticals], which doesn't exist." That leaves poorer countries vulnerable, he says, and keeps those in wealthier ones just satisfied enough that they don't feel the need to clamor for more dramatic action.
Plançon says Interpol is doing the best it can to divide its limited manpower between wealthy and poorer countries, while figuring out what unique approach each area will require. For example, on a recent visit to Guinea, Plançon helped authorities decide that the best use of their resources was to beef up their FDA equivalent instead of conducting a one-time bust of fake drugs.
Cellphone Salvation
The best way to catch a fake is to send samples to a lab where researchers can do tests. But even lab tests are not 100 percent accurate, and in low-income countries, sending thousands of samples to a lab is slow and prohibitively expensive. There are handheld mass spectrometers (a tool used to analyze the chemical makeup of foods, pharmaceuticals, etc.), but they are new, unproven and costly.
That's why scientists and tech innovators are working on getting cheap and effective solutions that can make a difference locally. The CD-3 is one promising example; it's a handheld device invented by the FDA that emits ultraviolet and infrared light onto pills and their packaging to determine if they are genuine. It's intuitive to use, relatively inexpensive at $1,000 per device and surprisingly effective.
In 2012, Patricia Tabernero, then a researcher at the Worldwide Antimalarial Resistance Network, a global project tracking falsified anti-malarials, was in Laos looking for fake drugs. At the time, Laos was on the brink of a health crisis because fake drugs were making diseases, especially malaria, more resistant; drugs with too little of the active ingredient kill some of the bacteria but leave the hardiest in the body to multiply and then spread.
Tabernero and her colleagues decided to try out the CD-3, in what would become the first field test of the device, in a developing country. First, they needed to collect sample drugs from as many pharmacies as possible in southern Laos. But they didn't want to tip off the pharmacists, who might skew their sample by giving the researchers more or fewer suspicious drugs, or even alert local criminals involved in manufacturing the fakes. So Tabernero and her European colleagues enlisted local volunteers to enter the pharmacies and ask, "I would like to buy some drugs for my friend who is sick. We are traveling and work in construction. May I see which ones you have?"
In just four weeks, Tabernero's team collected anti-malarials from 144 private drug outlets. In the evenings, she would test the samples in her hotel room using the CD-3. The results were encouraging: The local officials caught on quickly, mastering the device in just two days of training. And it worked well. They shipped all the tested pills to a U.S. Centers for Disease Control and Prevention lab for chemical analysis, to see how well the device did, and it was nearly 100 percent accurate for all the samples it tested.
But the CD-3 could evaluate only 35 percent of the samples. To see if a drug is legitimate, the instrument needs to compare it with a genuine example, which regulators may not have if the drug is rare or if the manufacturer changes the formula without warning. This is especially true in a developing country like Laos. So far, officials don't have access to a universal registry of packaging; if the CD-3 is going to become more popular and used worldwide, agencies like the FDA will need to form stronger partnerships with manufacturers to make this universal registry a reality.

In the meantime, other low-tech solutions are helping consumers in developing parts of the world protect themselves. The widespread use of cellphones has helped: Legitimate drug manufacturers are starting to design packaging with scratch-off codes that a consumer texts to a special phone number. They then receive an automatic response confirming whether or not the drug is genuine. This has promising initial results—so far, Herrington says, no falsifier has been able to hack this system. But in the long term, experts agree, this solution is not ideal because it doesn't strengthen the regulatory system, as the CD-3 does. And without a strong regulatory system, falsifiers can't be caught.
That's also why, ultimately, the problem will be fully solved only when large importing countries like the U.S. adopt stronger legislation and insist that their trade partners do the same. "If they don't want to play by the rules, within five years their access to the U.S. market is gone," Attaran says. Take India, for example. Indian manufacturers are currently responsible for 40 percent of the generic drugs in the U.S., and recently they have come under increased scrutiny due to lapses in quality and regulation. The FDA could place sanctions on the country or ban the import of all drugs "until India cleans up its act," Attaran says. The agency could provide a five-year grace period, he adds, to allow American drug companies to find trustworthy facilities and continue manufacturing to prevent drug shortages domestically.
The U.S. is not immune to fake drugs, of course. In 2012, hundreds of cancer patients took what they thought was Avastin, a monoclonal antibody, only to learn that their drug lacked the active ingredients. This past April, the FDA received reports of fake Botox in clinics all over the country. The FDA has a website warning consumers against fakes known to be in the drug supply. The government regularly files lawsuits against online pharmacies and their collaborators, charging them with drug trafficking, smuggling, counterfeiting and money laundering.
Nevertheless, one of the reasons the U.S. hasn't taken stronger international action is that most citizens don't know the problem is so pervasive. Americans enjoy some of the strongest regulatory and enforcement systems in the world. Despite the occasional problematic batch here and there, what consumers buy at their local pharmacy in the States is probably genuine, which means Americans are less likely to push our leaders to make changes.
Citizen awareness could make all the difference. It's happened before, most recently with dietary supplements with false claims about their ingredients or effects. For the past few years, people ranging from health nuts to journalists have decried the ineffective and largely unregulated supplements, which often contain ingredients not listed on the packaging. But enforcement agencies like the FDA didn't do anything about it. Earlier this year, enforcement agencies finally gave in; the New York attorney general, along with the FDA, began to crack down on supplement companies selling fake or dangerous products. In New York, authorities tested top-selling herbal supplements at four retail giants—GNC, Target, Walgreen and Wal-Mart—and found that 80 percent did not contain the medicinal herbs listed on the label. In response, attorneys for the state issues cease and desist letters to the retailers.
It's an elegant solution to a very complex problem: If citizens force the U.S. to play a larger role in the international conversation about falsified drugs, the drug supply would be safer within U.S. borders but would also extend far beyond. With the pressure on, countries would likely band together to share detection technologies, collaborate on a universal database of legitimate pharmaceuticals and pass international standards with real consequences. And that could quell rampant drug-resistant malaria in Laos, or save the lives of thousands of Sudanese citizens who thought they were safe because they got the meningitis vaccine but really were injected with little more than saline.